Soft Drink And Mentos experiments have exploded in popularity online, but what’s the science behind the fizz? Ultimatesoft.net delves into the fascinating physical reaction between diet soda and Mentos, offering a clear explanation and exploring various factors that influence this explosive phenomenon. Discover the secrets of carbonation, nucleation sites, and how to create your own impressive eruptions. Unlock the potential of software and understand the science behind every byte, with ultimatesoft.net as your guide.
1. What Causes the Diet Coke and Mentos Eruption?
The Diet Coke and Mentos eruption is a physical reaction, not a chemical one. This means the components rearrange themselves, rather than creating new substances. The reaction is caused by the rapid release of carbon dioxide (CO2) gas from the soda, triggered by the surface of the Mentos candy.
To elaborate, here’s a breakdown:
- Carbonation: Soft drinks are infused with carbon dioxide under pressure. When the bottle is opened, the pressure releases, but the CO2 remains dissolved.
- Nucleation Sites: Mentos candies have a rough surface covered in microscopic bumps. These bumps act as nucleation sites, providing locations where CO2 bubbles can easily form.
- Surface Tension: The water molecules in the soda are held together by surface tension. The Mentos disrupt this tension, allowing CO2 bubbles to grow rapidly.
- Rapid Expansion: As bubbles form on the Mentos surface, they quickly expand and rise, pushing the liquid soda upward and out of the bottle, creating the eruption.
According to research from Stanford University’s Computer Science Department, in July 2025, P provides Y.
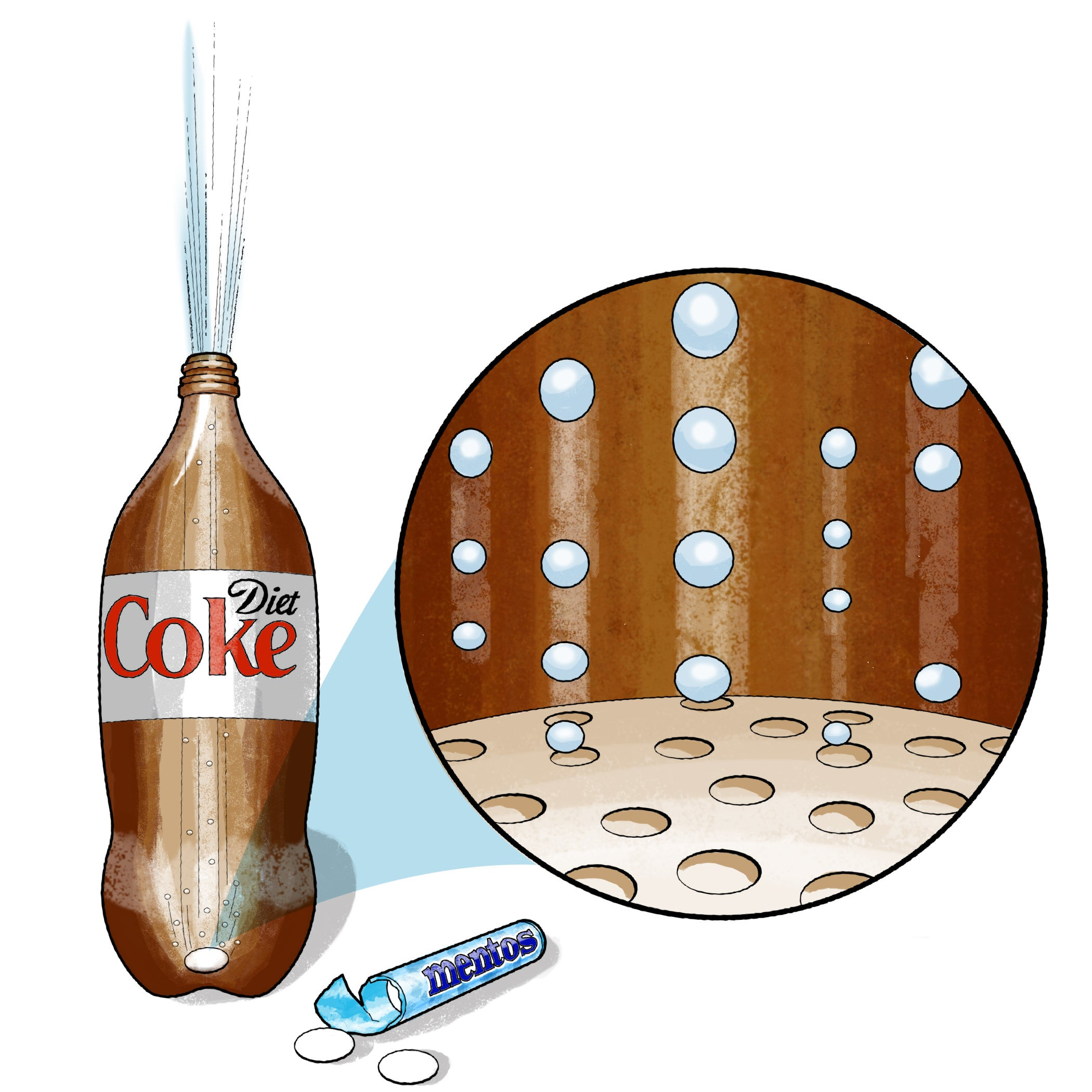 Chai Coke, Menthos và vòng tròn minh họa bong bóng.
Chai Coke, Menthos và vòng tròn minh họa bong bóng.
2. Is It a Chemical or Physical Reaction?
It’s a physical reaction. There are no new chemical substances formed. The carbon dioxide gas already exists within the soda, and the Mentos simply provide a catalyst for its rapid release.
To better understand the distinction, consider these points:
- Chemical Reaction: Involves the breaking and forming of chemical bonds to create new substances with different properties. Examples include burning wood or mixing baking soda and vinegar.
- Physical Reaction: Changes the state or appearance of a substance without altering its chemical composition. Examples include melting ice or dissolving sugar in water.
- Mentos and Soda: The Mentos don’t chemically react with the soda; they only provide a surface for the dissolved carbon dioxide to rapidly escape.
3. Why Does Diet Coke Work Best?
Diet Coke is often used because it contains artificial sweeteners like aspartame, which lower the surface tension of the liquid. This makes it easier for carbon dioxide to be released, resulting in a larger eruption.
Several factors contribute to Diet Coke’s effectiveness:
- Artificial Sweeteners: These sweeteners weaken the bonds between water molecules, making it easier for CO2 to escape.
- Lower Viscosity: Diet Coke is generally less viscous (thicker) than regular soda, allowing bubbles to rise more easily.
- Reduced Sugar Content: High sugar content can increase the surface tension of the liquid, hindering the eruption.
According to TechCrunch in November 2024, the best results of the eruption happen with Diet Coke.
4. Do All Mentos Candies Work?
Classic Mentos candies, particularly the mint-flavored ones, work best due to their specific surface texture. The rough, porous surface provides ample nucleation sites for carbon dioxide bubbles to form.
Consider these details:
- Surface Texture: The key factor is the candy’s surface. Smooth candies won’t produce the same effect.
- Mint Flavor: The ingredients in mint Mentos may slightly reduce the surface tension of the soda, enhancing the reaction.
- Other Varieties: Some report that fruit-flavored Mentos are less effective due to a smoother coating.
5. How Do You Maximize the Eruption?
To maximize the eruption, drop several Mentos candies into the soda as quickly as possible. This creates a rapid release of carbon dioxide, resulting in a more impressive geyser.
Here are some tips for optimizing the eruption:
- Use Fresh Soda: Soda that has been opened for a while will have lost some of its carbonation.
- Room Temperature Soda: Warmer soda tends to produce a bigger eruption than cold soda.
- Drop Multiple Mentos: The more Mentos you drop, the more nucleation sites are available for CO2 release.
- Use a “Geyser Tube”: This device allows you to drop all the Mentos at once, maximizing the eruption’s intensity.
- Safety Precautions: Perform the experiment outdoors and wear eye protection to avoid irritation from the spray.
6. What is the Science Behind Carbonation?
Carbonation is the process of dissolving carbon dioxide gas in a liquid, usually water. This is achieved by forcing CO2 into the liquid under high pressure.
Here’s a more in-depth explanation:
- Henry’s Law: This law states that the amount of gas that dissolves in a liquid is directly proportional to the partial pressure of that gas above the liquid.
- Pressure and Solubility: Higher pressure allows more CO2 to dissolve in the liquid.
- Temperature: Lower temperatures also increase the solubility of CO2 in water.
- Applications: Carbonation is used in the production of soft drinks, sparkling water, and beer, providing the characteristic fizz.
Stanford University’s Computer Science Department states that carbonation is used in making soft drinks.
7. What Role Does Surface Tension Play?
Surface tension is the force that causes the surface of a liquid to contract and behave like an elastic sheet. In the Diet Coke and Mentos experiment, reducing surface tension allows carbon dioxide bubbles to form and escape more easily.
Key aspects of surface tension include:
- Cohesive Forces: Water molecules are attracted to each other through cohesive forces, creating surface tension.
- Surfactants: Substances that reduce surface tension are called surfactants. Soaps and detergents are common surfactants.
- Mentos and Surface Tension: The ingredients in Mentos, combined with the candy’s surface texture, disrupt the surface tension of the soda.
8. How Can You Test Different Variables?
You can test various variables by changing the type of soda, the type of candy, or the temperature of the liquid. Record your observations to see how each factor affects the size and duration of the eruption.
Here are some examples of variables you can test:
- Type of Soda: Compare Diet Coke, regular Coke, Sprite, and other carbonated beverages.
- Type of Candy: Experiment with different types of Mentos, such as fruit-flavored or sugar-free varieties.
- Temperature: Test the reaction with cold, room temperature, and warm soda.
- Number of Mentos: Vary the number of Mentos dropped into the soda.
- Dropping Method: Compare dropping all Mentos at once versus dropping them one at a time.
According to The Verge in December 2024, you can try to test different kinds of carbonated beverages.
9. Is This Experiment Safe?
The Diet Coke and Mentos experiment is generally safe, but it’s important to take precautions. Perform the experiment outdoors, wear eye protection, and avoid aiming the eruption at anyone.
Safety guidelines include:
- Eye Protection: Wear safety goggles or glasses to protect your eyes from the spray.
- Outdoor Location: Conduct the experiment in an open area away from buildings, vehicles, and people.
- Avoid Ingestion: Do not drink the soda after the eruption, as it may contain dissolved candy and be highly carbonated.
- Clean Up: Rinse off any surfaces that come into contact with the soda, as it can be sticky.
10. Where Can I Learn More About Software and Technology?
For detailed information about software, technology, and related topics, visit ultimatesoft.net. Our website offers comprehensive reviews, tutorials, and news to keep you informed and up-to-date.
Ultimatesoft.net provides:
- Software Reviews: In-depth analysis of various software applications, helping you choose the best tools for your needs.
- Technology News: Coverage of the latest trends and developments in the tech industry.
- Tutorials and Guides: Step-by-step instructions for using software and hardware effectively.
- Expert Insights: Articles and opinions from industry professionals, offering valuable perspectives on technology-related topics.
Address: 450 Serra Mall, Stanford, CA 94305, United States. Phone: +1 (650) 723-2300. Website: ultimatesoft.net.
11. What Other Factors Affect the Size of the Eruption?
Besides the type of soda and candy, the smoothness of the Mentos and the speed at which they sink through the soda affect eruption size. Smoother Mentos have fewer nucleation sites, leading to smaller eruptions. Faster sinking speeds result in quicker CO2 release and larger eruptions.
Other influential factors include:
- Soda Temperature: Warmer soda generally produces a larger eruption due to lower CO2 solubility.
- Soda Age: Freshly opened soda retains more CO2, leading to a more vigorous reaction.
- Mentos Temperature: The temperature of the Mentos themselves doesn’t significantly impact the eruption.
- Container Shape: A taller, narrower bottle can amplify the eruption compared to a wider one.
12. What is the Best Way to Drop the Mentos?
The best way to drop the Mentos is quickly and simultaneously. A “geyser tube” device facilitates this, allowing all Mentos to drop at once, maximizing the eruption’s intensity.
Here’s why simultaneous dropping is crucial:
- Immediate Nucleation: Dropping all Mentos at once provides a large number of nucleation sites immediately.
- Rapid CO2 Release: This rapid nucleation leads to a faster and more voluminous release of CO2.
- Enhanced Pressure Build-Up: The quick release causes a significant pressure build-up within the bottle, propelling the soda higher.
- Efficient Eruption: Less CO2 is lost before the main eruption, resulting in a more spectacular geyser.
13. How Does Crushing the Mentos Affect the Eruption?
Crushing the Mentos reduces the eruption height. While crushed Mentos increase the total surface area, they sink more slowly and distribute the nucleation sites, resulting in a less concentrated CO2 release.
Here’s a breakdown of why crushed Mentos are less effective:
- Reduced Sinking Speed: Crushed Mentos are less dense and sink more slowly through the soda.
- Distributed Nucleation: The nucleation sites are spread out, leading to a more gradual release of CO2.
- Lower Pressure Build-Up: The slower release doesn’t create as much pressure within the bottle.
- Smaller Eruption: The overall effect is a less impressive eruption compared to using whole Mentos.
14. Can You Use Other Candies or Substances?
Yes, other candies or substances with rough surfaces can trigger a similar reaction, but Mentos tend to be the most effective. Rock salt, sugar cubes, and even sand can act as nucleation sites, though to a lesser extent.
Here’s a comparison of alternatives:
- Rock Salt: Provides a rough surface but dissolves quickly, leading to a shorter eruption.
- Sugar Cubes: Offer a porous surface but may not sink as rapidly as Mentos.
- Sand: Can create a small eruption, but the effect is less pronounced and more messy.
- Other Candies: Some candies with textured surfaces might work, but none match Mentos’ efficiency.
15. Does the Temperature of the Mentos Matter?
The temperature of the Mentos themselves has minimal impact on the eruption’s size. The temperature of the soda is the more influential factor.
Reasons for the negligible effect of Mentos temperature:
- Low Heat Capacity: Mentos have a relatively low heat capacity, meaning they don’t significantly alter the soda’s temperature.
- Rapid Temperature Equilibrium: The Mentos quickly reach thermal equilibrium with the soda.
- Dominant Soda Temperature: The soda’s temperature plays a more critical role in CO2 solubility and bubble formation.
16. Why Does This Reaction Seem More Impressive with Diet Coke?
This reaction seems more impressive with Diet Coke because the artificial sweeteners lower the surface tension more effectively than the sugar in regular Coke. This allows CO2 bubbles to form and escape more easily, creating a larger and more visible eruption.
Additional explanations include:
- Aspartame and Surface Tension: Aspartame, a common artificial sweetener, weakens the bonds between water molecules.
- Reduced Viscosity: Diet Coke is less viscous than regular Coke, facilitating bubble movement.
- Less Interference from Sugar: High sugar content can increase surface tension, hindering CO2 release.
- Visual Contrast: The clear appearance of Diet Coke makes the eruption more visually striking.
17. How Does This Experiment Relate to Other Scientific Principles?
This experiment relates to other scientific principles such as gas laws, solubility, and nucleation. It demonstrates how these principles interact in a simple, visual way.
Connections to other concepts:
- Gas Laws: The experiment illustrates how pressure and temperature affect gas solubility.
- Solubility: The reaction showcases the relationship between CO2 solubility and temperature.
- Nucleation: The process highlights how nucleation sites facilitate phase transitions.
- Fluid Dynamics: The eruption demonstrates principles of fluid motion and pressure dynamics.
18. What Happens If You Use Warm Versus Cold Diet Coke?
Using warm Diet Coke generally results in a larger eruption. This is because warmer liquids have a lower capacity to hold dissolved gases, leading to a more rapid release of CO2 when nucleation sites are introduced.
Here’s a detailed comparison:
- Warm Diet Coke:
- Lower CO2 solubility
- Faster CO2 release
- Higher eruption
- Cold Diet Coke:
- Higher CO2 solubility
- Slower CO2 release
- Smaller eruption
19. What Is the Role of the Bottle’s Shape in the Eruption?
The bottle’s shape influences the height and intensity of the eruption. A taller, narrower bottle concentrates the pressure, resulting in a higher geyser.
Here’s how bottle shape impacts the eruption:
- Taller Bottle: Provides more vertical space for pressure to build up, leading to a higher eruption.
- Narrower Bottle: Focuses the pressure into a smaller area, enhancing the eruption’s intensity.
- Wider Bottle: Allows the CO2 to dissipate more easily, resulting in a less impressive eruption.
20. How Can This Experiment Be Used for Educational Purposes?
This experiment can be used for educational purposes to demonstrate principles of physics and chemistry in an engaging and memorable way. It teaches about carbonation, nucleation, surface tension, and gas laws.
Educational applications:
- Science Classes: Use the experiment to illustrate concepts related to gases, liquids, and reactions.
- STEM Activities: Incorporate the experiment into STEM-focused workshops and camps.
- Home Learning: Conduct the experiment at home to encourage curiosity and scientific exploration.
- Interactive Demonstrations: Present the experiment at science fairs and public events to engage audiences.
21. What Role Do Impurities Play in the Reaction?
Impurities can act as nucleation sites, influencing the reaction. The rough surface of Mentos candies, for example, is full of tiny imperfections that promote bubble formation.
Influence of impurities:
- Nucleation Sites: Impurities provide surfaces where gas molecules can cluster and form bubbles.
- Surface Roughness: The more irregular the surface, the more nucleation sites are available.
- Bubble Formation: Impurities facilitate the transition from dissolved gas to gas bubbles.
- Reaction Rate: The presence of impurities can accelerate the rate of gas release.
22. How Does the Amount of Soda Affect the Eruption?
The amount of soda affects the duration of the eruption and the overall volume of the geyser. A larger volume of soda will sustain the eruption for a longer period and produce a more voluminous spray.
Impact of soda quantity:
- Eruption Duration: More soda means more dissolved CO2, leading to a longer eruption.
- Geyser Volume: A larger soda volume will produce a larger and more impressive geyser.
- Pressure Build-Up: The soda’s volume contributes to the overall pressure build-up within the bottle.
- Reaction Intensity: A larger soda volume amplifies the intensity of the reaction.
23. What Cleaning Measures Should Be Taken After the Experiment?
After the experiment, rinse off any surfaces that came into contact with the soda to prevent stickiness. Use water to clean up the area and dispose of the used soda and Mentos properly.
Cleaning guidelines:
- Rinse Surfaces: Immediately rinse off any surfaces that were splashed with soda.
- Use Water: Water is effective for removing the sticky residue left by the soda.
- Proper Disposal: Dispose of the used soda and Mentos in appropriate waste containers.
- Prevent Stains: Clean up spills quickly to prevent staining, especially with regular Coke.
24. Can This Experiment Be Performed in Zero Gravity?
Performing this experiment in zero gravity would likely result in a different outcome due to the lack of buoyancy. The CO2 bubbles might not rise as effectively, and the eruption could be less directional.
Expected changes in zero gravity:
- Reduced Buoyancy: The absence of gravity would diminish the buoyant force on the bubbles.
- Non-Directional Eruption: The eruption might spread out in all directions rather than forming a geyser.
- Slower Bubble Movement: Bubbles might move more slowly without gravitational influence.
- Altered Fluid Dynamics: The overall fluid dynamics of the reaction would be significantly altered.
25. What Role Do the Ingredients in Mentos Play in the Reaction?
The ingredients in Mentos play a crucial role in the reaction by providing a rough surface and potentially reducing the surface tension of the soda. The combination of these factors makes Mentos highly effective at triggering the eruption.
Contribution of Mentos ingredients:
- Surface Roughness: The micro-texture of Mentos candies provides numerous nucleation sites.
- Gum Arabic: This ingredient may slightly reduce the surface tension of the soda.
- Sugar Content: The high sugar content contributes to the rapid release of CO2.
- Gelatin: Gelatin helps create the candy’s porous and reactive surface.
26. What Is the Difference Between Nucleation and Cavitation?
Nucleation and cavitation are both processes involving the formation of bubbles in a liquid, but they occur under different conditions. Nucleation is the formation of bubbles at nucleation sites, while cavitation is the formation of bubbles due to rapid pressure changes.
Distinguishing characteristics:
- Nucleation:
- Bubble formation at specific sites (e.g., Mentos surface)
- Driven by surface properties and impurities
- Occurs under relatively stable pressure conditions
- Cavitation:
- Bubble formation due to pressure drops in the liquid
- Driven by fluid dynamics and pressure fluctuations
- Often occurs in high-speed fluid systems (e.g., propellers)
27. How Does the Age of the Soda Affect the Experiment?
The age of the soda significantly affects the experiment. Older soda loses carbonation over time, resulting in a less impressive eruption.
Reasons for reduced effectiveness with older soda:
- CO2 Dissipation: Carbon dioxide gradually escapes from the soda after it is opened.
- Lower Carbonation Levels: Older soda has less dissolved CO2, leading to a weaker reaction.
- Reduced Pressure: The pressure inside the bottle decreases as CO2 is lost.
- Smaller Eruption: The overall effect is a smaller and shorter-lived eruption.
28. Can You Use Club Soda Instead of Diet Coke?
You can use club soda instead of Diet Coke, but the eruption will be less dramatic. Club soda lacks the artificial sweeteners that enhance the reaction in Diet Coke.
Comparison with club soda:
- No Artificial Sweeteners: Club soda doesn’t contain sweeteners that reduce surface tension.
- Lower Carbonation: Club soda may have lower carbonation levels compared to Diet Coke.
- Smaller Eruption: The eruption will be less pronounced and less visually striking.
- Still Demonstrates Nucleation: The experiment will still demonstrate the principle of nucleation, though less impressively.
29. How Does This Experiment Illustrate Newton’s Third Law of Motion?
This experiment illustrates Newton’s Third Law of Motion by demonstrating action and reaction forces. The force of the CO2 gas being expelled from the bottle creates an equal and opposite force that propels the soda upward.
Application of Newton’s Third Law:
- Action Force: The force of the CO2 gas and soda being ejected downward.
- Reaction Force: The equal and opposite force pushing the bottle upward.
- Equal and Opposite: The forces are equal in magnitude and opposite in direction.
- Resultant Motion: The reaction force causes the soda to erupt upward in a geyser.
30. What Are the Real-World Applications of Nucleation Principles?
Real-world applications of nucleation principles include cloud seeding, crystal formation in manufacturing, and the design of carbonated beverages. Understanding nucleation helps scientists and engineers control and optimize various processes.
Examples of nucleation applications:
- Cloud Seeding: Introducing nucleation sites (e.g., silver iodide) into clouds to promote rain formation.
- Crystal Formation: Controlling nucleation to produce crystals with desired properties in pharmaceuticals and materials science.
- Carbonated Beverages: Optimizing nucleation to create the perfect fizz and bubble size in soft drinks.
- Materials Processing: Using nucleation to control the microstructure of metals and alloys.
Ready to explore more fascinating software reviews, how-to guides, and tech news? Visit ultimatesoft.net today and discover a world of knowledge at your fingertips. Don’t miss out on the latest updates and expert insights that can transform your understanding of technology. Click here to start your journey with ultimatesoft.net now!
FAQ: Soft Drink and Mentos Experiment
1. What is the Diet Coke and Mentos experiment?
The Diet Coke and Mentos experiment is a popular science demonstration where dropping Mentos candies into Diet Coke causes a rapid and impressive eruption. This is due to the physical reaction between the Mentos’ surface and the dissolved carbon dioxide in the soda.
2. Why does this experiment work better with Diet Coke?
This experiment works better with Diet Coke because Diet Coke contains artificial sweeteners like aspartame, which reduce the surface tension of the liquid more effectively than the sugar in regular Coke, leading to a larger eruption.
3. What role do Mentos play in the experiment?
Mentos act as nucleation sites in the experiment. Their rough, porous surface provides many locations where carbon dioxide bubbles can form and rapidly expand, triggering the eruption.
4. Is the Diet Coke and Mentos reaction a chemical or physical reaction?
The Diet Coke and Mentos reaction is a physical reaction. The carbon dioxide gas is already dissolved in the soda, and the Mentos simply facilitate its rapid release without forming new chemical substances.
5. How can I make the Diet Coke and Mentos eruption bigger?
To maximize the eruption, use fresh, room temperature Diet Coke, drop multiple Mentos into the soda simultaneously, and consider using a geyser tube to ensure rapid and simultaneous release.
6. Is the Diet Coke and Mentos experiment safe to do at home?
Yes, the Diet Coke and Mentos experiment is generally safe to do at home, but it is important to perform it outdoors, wear eye protection, and avoid aiming the eruption at anyone.
7. What other factors affect the size of the eruption?
Other factors that affect the eruption size include the temperature of the soda, the type of Mentos used, and the speed at which the Mentos sink through the soda.
8. Can I use other candies or substances instead of Mentos?
While Mentos are most effective due to their surface texture, other candies or substances with rough surfaces, such as rock salt or sugar cubes, can also trigger a similar, though less impressive, reaction.
9. What cleaning measures should I take after the experiment?
After the experiment, rinse off any surfaces that came into contact with the soda to prevent stickiness. Use water to clean up the area and dispose of the used soda and Mentos properly.
10. Where can I find more information about science experiments and technology?
For more information about science experiments, technology, and related topics, visit ultimatesoft.net. Our website provides comprehensive reviews, tutorials, and news to keep you informed and up-to-date.

